Heading 1
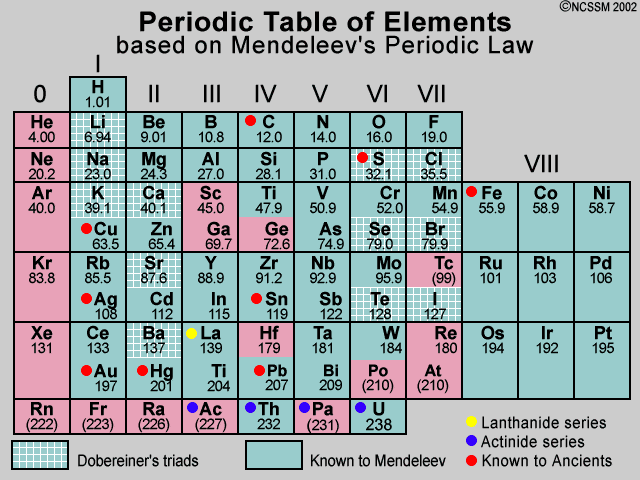
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character (keeping their own electrons) increasing from left to right across a period, and from down to up across a group, and metallic character (surrendering electrons to other atoms) increasing in the opposite direction. The underlying reason for these trends is electron configurations of atoms. The periodic table exclusively lists electrically neutral atoms that have an equal number of positively charged protons and negatively charged electrons and puts isotopes (atoms with the same number of protons but different numbers of neutrons) at the same place. Other atoms, like nuclides and isotopes, are graphically collected in other tables like the tables of nuclides (often called Segrè charts).
11 DEC
Heading 2
The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869: he formulated the periodic law as a dependence of chemical properties on atomic mass. Because not all elements were then known, there were gaps in his periodic table, and Mendeleev successfully used the periodic law to predict properties of some of the missing elements. The periodic law was recognized as a fundamental discovery in the late 19th century, and it was explained with the discovery of the atomic number and pioneering work in quantum mechanics of the early 20th century that illuminated the internal structure of the atom. With Glenn T. Seaborg's 1945 discovery that the actinides were in fact f-block rather than d-block elements, a recognisably modern form of the table was reached. The periodic table and law are now a central and indispensable part of modern chemistry.
11 DEC

Heading 3
The periodic table continues to evolve with the progress of science. In nature, only elements up to atomic number 94 exist; to go further, it was necessary to synthesise new elements in the laboratory. Today, all the first 118 elements are known, completing the first seven rows of the table, but chemical characterisation is still needed for the heaviest elements to confirm that their properties match their positions. It is not yet known how far the table will stretch beyond these seven rows and whether the patterns of the known part of the table will continue into this unknown region. Some scientific discussion also continues regarding whether some elements are correctly positioned in today's table. Many alternative representations of the periodic law exist, and there is some discussion as to whether there is an optimal form of the periodic table.
11 DEC

Heading 4
Overview
11 DEC